New types of electronic and magnetic functions created by arranging functional molecule units or by reducing materials size to nano-dimension
We can create unconventional properties which existing bulk materials do not have, by fractionalizing materials into nanosized systems having one, two, and three dimensional structures, such as ultra-thin films, nanowires, and nanoparticles. In the meantime, we can happen to create unconventional functions by assembling molecules or molecule-sized building blocks into nano-architectures as a consequence of the interaction acting between the units. In the cutting-edge nanotechnology/nanoscience, we have recognized the importance of the nano-architecture created by the arrangement of molecules/atoms, or by reducing the size of materials to nano-dimension (1 nm=10-9 m). In our group, we have been challenging to extract the electronic functions of a molecule by observing the conductivity of a single molecule, and to create carbon-based nano-architectures and a variety of their unconventional electronic and magnetic properties. Multifunctional molecular systems created by assembling molecule units having different functions are also targets of our challenge.
New classes of carbon nanostructures and their unconventional electronic and magnetic properties
We have a large variety of carbon allotropes having different electronic properties, as we know from the fact that diamond is an insulator with extreme mechanical hardness, whereas graphite is an electrical conductor with layered structure which can be easily cleaved. This variety originates from the variety of C-C bonding manners, such as single, double and triple bondings. Fullerenes such as C60, carbon nanotubes, and graphene (single sheet of graphite) are also involved in the carbon allotropes. We have been challenging to create nanosized graphene (nanographene) and to clarify its electronic and magnetic properties. The electron in a nanographene sheet has behavior entirely different from that in conventional conducting materials, as a consequence of quantum effect and geometrical effect on the electrons confined in the nanosized two dimensional sheet. From chemistry aspect, the electronic structure of nanographene can be understand along the line of structural organic chemistry, as it is what is extrapolated from condensed polycyclic hydrocarbon molecules such as naphthalene, anthracene to nanodimension. The development of the interdisciplinary frontier between condensed matter physics and structural organic chemistry is our challenge in the research on nanographene.
We are interested also in carbon nanomaterials with their structures intermediate between diamond and graphite, and nanographene-based nanoporous materials. Guest species confined into the nanospace not only have structures entirely different from those in the bulk state, but also show unconventional electronic and magnetic properties.
 |
 |
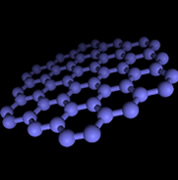 |
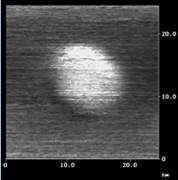
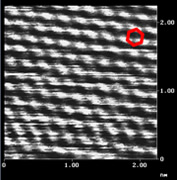
Nanographene sheet with a size of 10 nm (left) and its lattice image (center), observed by scanning tunneling microscopy, and schematic model of nanographene (right). |
||
 |
||
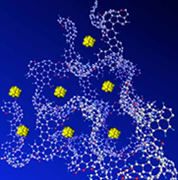
Alkali metal clusters regularly arranged in a nanopore space of carbon nanomaterial. |
||
Single molecular junction
When a single molecule is bridged between metal electrodes, the single molecule could show interesting phenomena which can not be observed in its bulk phase. The single molecular junction has also attracted wide attention due to its potential application for future electronic devices. We have been challenging to fabricate single molecular junctions using variety of molecules and to investigate their electrical conductivity. We are also challenging to develop the vibration spectroscopy of a single molecular junction to see the single molecule bridging between metal electrode, and to control the properties of the single molecular junction by applying the external field. Our final goal is to reveal the unique properties which are characteristic of single molecular junctions.
 |
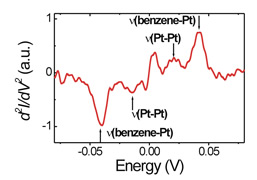 |
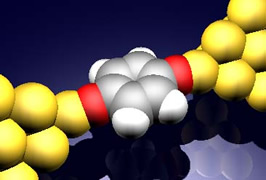
Single molecular junction (left), Vibration spectroscopy of a single benzene molecule bridging between Pt electrodes (right) |
|
Microprobe observations and investigations of nanostructures in the atomic resolution
Scanning Probe Microprobe (SPM) techniques, which are based on electron tunneling phenomenon and forces created by chemical interactions, can offer direct information of atomic arrangements and electronic structure of nanostructures in the atomic resolution. We have been investigating the geometrical atomic arrangements and the local electronic structures of nanographene, graphene edges, and nanostructures created by the reaction of nanographene with chemical species such as oxygen, by means of scanning tunneling microscopy/spectroscopy and atomic force microscopy. Microprobe investigations at low temperature and under strong magnetic field give information on the detailed structures and can unveil unconventional behavior of the electrons confined into the nanospace.
 |
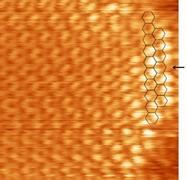 |
Low temperature scanning tunneling microscope with a high field magnet (left). Electron confinement in a finite size graphene zigzag edge observed by scanning tunneling microscope (right). |
New functional materials in molecule-based architecture
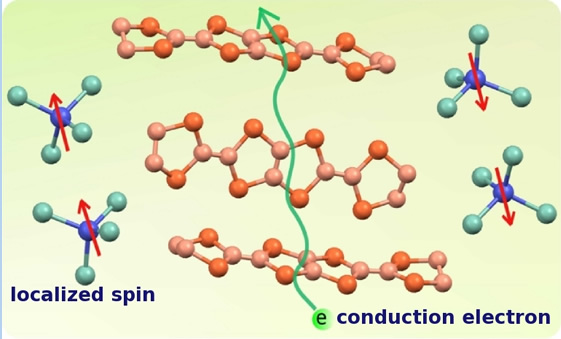 |
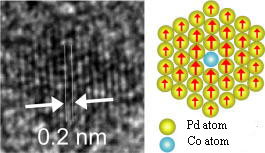 |